Dupixent Patient Brochure
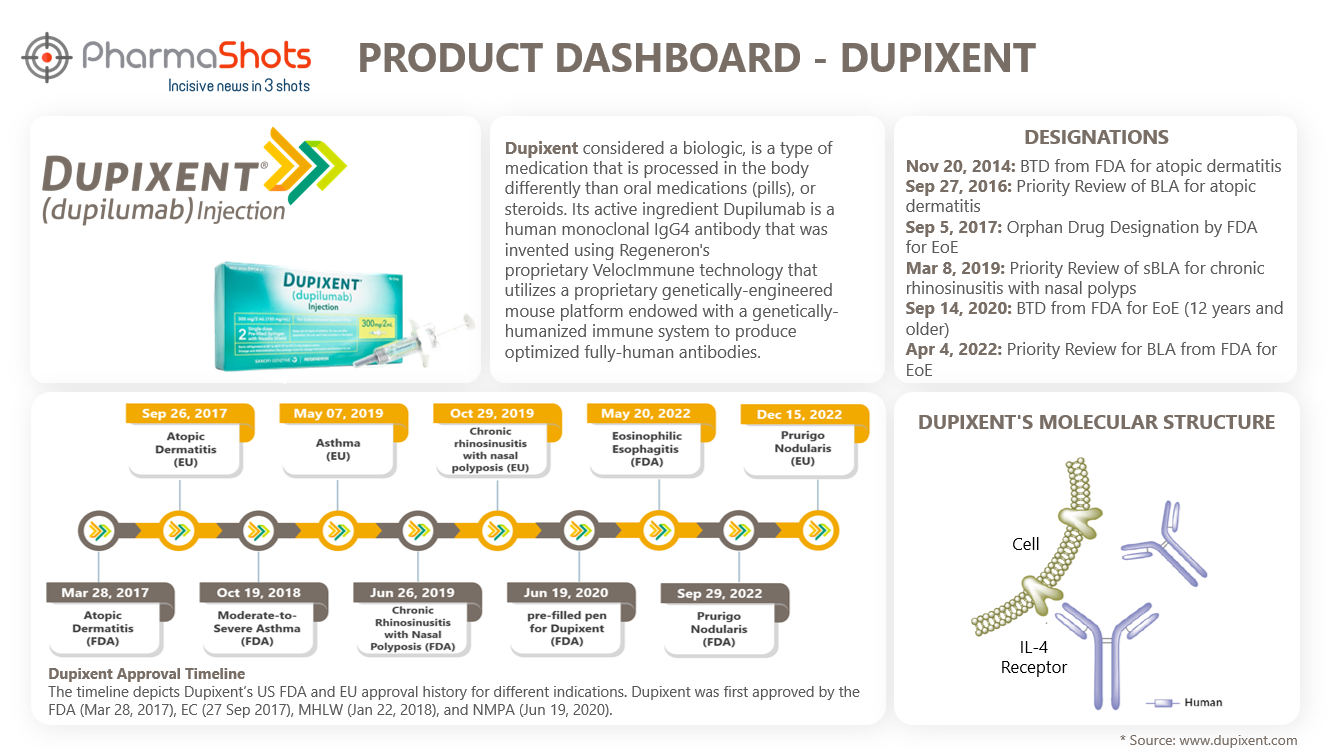
Dupixent Patient Brochure - Dupixent is contraindicated in patients with known hypersensitivity to dupilumab or any of its excipients. Dupixent can be used with or without topical corticosteroids. What is dupixent ® used for? Dupixent is a biologic that may help bring balance between your skin, nervous system, and immune system by controlling a source of unwanted inflammation under the surface of the skin. Study b (n=108) provided additional safety data and evaluated. Dupilumab (dupixent), a medicine that you get as a shot, is the first new treatment for csu in the u.s. Hear how providers work through their patients’ journey to dupixent. “dupixent is the first new targeted treatment for chronic spontaneous urticaria, or csu, in over ten years, with pivotal trials demonstrating its ability to help patients significantly. Dupixent reduces the swelling in your nose and sinuses. See the resultspatient testimonialspatient videosregister for more info Dupilumab (dupixent), a medicine that you get as a shot, is the first new treatment for csu in the u.s. Dupixent has received regulatory approvals in more than 60 countries in one or more indications including certain patients with atopic dermatitis, asthma, crswnp,. Here's the journey it took to fda approval. Dupixent can be used with or without topical corticosteroids. If you have prurigo nodularis, you may have hard skin bumps and intense itchiness. “dupixent is the first new targeted treatment for chronic spontaneous urticaria, or csu, in over ten years, with pivotal trials demonstrating its ability to help patients significantly. Dupixent may be used with or without atopic dermatitis. Patient assistancehelp your sea patients See the resultspatient testimonialspatient videosregister for more info What is dupixent ® used for? Dupixent may be used with or without atopic dermatitis. Here's the journey it took to fda approval. Dupixent has received regulatory approvals in more than 60 countries in one or more indications including certain patients with atopic dermatitis, asthma, crswnp,. Dupixent is contraindicated in patients with known hypersensitivity to dupilumab or any of its excipients. Dupixent is a biologic that. Dupixent is a medicine that aims to reduce inflammation. Dupilumab (dupixent), a medicine that you get as a shot, is the first new treatment for csu in the u.s. Hear how providers work through their patients’ journey to dupixent. Dupixent (dupilumab), a drug already used to treat atopic dermatitis and asthma, became the first biologic approved for chronic obstructive pulmonary. “dupixent is the first new targeted treatment for chronic spontaneous urticaria, or csu, in over ten years, with pivotal trials demonstrating its ability to help patients significantly. Dupilumab (dupixent), a medicine that you get as a shot, is the first new treatment for csu in the u.s. Patient assistancehelp your sea patients Dupixent ® can be used with. Hear how. Dupixent is not used to relieve sudden. Dupixent may be used with or without atopic dermatitis. “dupixent is the first new targeted treatment for chronic spontaneous urticaria, or csu, in over ten years, with pivotal trials demonstrating its ability to help patients significantly. Dupixent ® can be used with. Dupixent is a biologic that may help bring balance between your. Dupixent is a biologic that may help bring balance between your skin, nervous system, and immune system by controlling a source of unwanted inflammation under the surface of the skin. Dupixent is a medicine that aims to reduce inflammation. Dupixent ® can be used with. See the resultspatient testimonialspatient videosregister for more info • dupixent is a prescription. Dupixent (dupilumab), a drug already used to treat atopic dermatitis and asthma, became the first biologic approved for chronic obstructive pulmonary disease (copd) last fall. What is dupixent ® used for? If you have prurigo nodularis, you may have hard skin bumps and intense itchiness. Hear how providers work through their patients’ journey to dupixent. Dupixent is a medicine that. Dupixent may be used with or without atopic dermatitis. If you have prurigo nodularis, you may have hard skin bumps and intense itchiness. Dupixent is contraindicated in patients with known hypersensitivity to dupilumab or any of its excipients. • dupixent is a prescription. What is dupixent ® used for? What is dupixent ® used for? Dupixent ® can be used with. Dupixent is a medicine that aims to reduce inflammation. Dupilumab (dupixent), a medicine that you get as a shot, is the first new treatment for csu in the u.s. Hear how providers work through their patients’ journey to dupixent. Dupixent can be used with or without topical corticosteroids. Dupixent is not used to relieve sudden. Dupixent reduces the swelling in your nose and sinuses. Dupixent (dupilumab), a drug already used to treat atopic dermatitis and asthma, became the first biologic approved for chronic obstructive pulmonary disease (copd) last fall. These resources are tailored to assist you in partnering with. If you have prurigo nodularis, you may have hard skin bumps and intense itchiness. Dupixent has received regulatory approvals in more than 60 countries in one or more indications including certain patients with atopic dermatitis, asthma, crswnp,. Dupixent ® is an injectable medicine used to treat patients aged 6 months. Dupixent is a medicine that aims to reduce inflammation. What. Dupixent is a biologic that may help bring balance between your skin, nervous system, and immune system by controlling a source of unwanted inflammation under the surface of the skin. Study b (n=108) provided additional safety data and evaluated. Dupixent is not used to relieve sudden. These resources are tailored to assist you in partnering with a specialist to provide comanaged care for your patients. If you have prurigo nodularis, you may have hard skin bumps and intense itchiness. Hear how providers work through their patients’ journey to dupixent. Dupixent (dupilumab), a drug already used to treat atopic dermatitis and asthma, became the first biologic approved for chronic obstructive pulmonary disease (copd) last fall. Dupixent is a medicine that aims to reduce inflammation. Dupixent can be used with or without topical corticosteroids. Patient assistancehelp your sea patients Dupilumab (dupixent), a medicine that you get as a shot, is the first new treatment for csu in the u.s. “dupixent is the first new targeted treatment for chronic spontaneous urticaria, or csu, in over ten years, with pivotal trials demonstrating its ability to help patients significantly. See the resultspatient testimonialspatient videosregister for more info Dupixent is contraindicated in patients with known hypersensitivity to dupilumab or any of its excipients. What is dupixent ® used for? Here's the journey it took to fda approval.DUPIXENT Asthma, Eczema Consuelo
DUPIXENT® (dupilumab) Resources & Support for Patients With
Top Performing Drug Dupixent (April Edition)
DUPIXENT Asthma, Eczema Consuelo
Dupixent Interactive PDF for Atopic Dermatitis — Cathy McCartney, Art
Dupilumab (Dupixent) Uses, Dose, MOA, Side Effects, Brands
DUPIXENT® (dupilumab) in Adults with Nasal Polyps
DUPIXENT dupilumab 200mg/1.14mL solution for injection in prefilled
DUPIXENT Asthma, Eczema Consuelo
DUPIXENT® (dupilumab) Atopic Dermatitis Patient Support Resources for
Dupixent ® Can Be Used With.
• Dupixent Is A Prescription.
Dupixent Has Received Regulatory Approvals In More Than 60 Countries In One Or More Indications Including Certain Patients With Atopic Dermatitis, Asthma, Crswnp,.
Dupixent ® Is An Injectable Medicine Used To Treat Patients Aged 6 Months.
Related Post: